22+ ionic or covalent calculator
There are 3 different types. Because most metals form cations and.

Pgnminadi8 Q M
If the difference is 2 it is an.

. Binary compounds compounds comprising two. So far in this text especially in section 42 we have described the two idealized extremes of chemical bonding. Ionic Calculator is a free online tool that displays the Ionic Formula of an Ionic Compound in a fraction of seconds on providing the inputs.
The Covalent Ionic Resonance Energy is the kinetic energy produced as a resultant of large participation or orbitals or covalentionic mixing and is represented as Δ EA-B-EA-B cov or. The best way to tell if the compound is ionic or covalent As a general rule of thumb compounds that involve a metal binding with either a non-metal or a semi-metal will display. The net ionic charge is nothing but the number of cations or anions a chemical compound is having.
Ionic vs Covalent Bonds. 1 ionic bondingin which one or more electrons are transferred. If one of the atom is electronegative it has more tendency to attract the electrons.
Percent ionic character is a measure of compound or molecules ionic and covalent character. If the electronegativity difference is between 04 and 200 the bond is polar covalent. Then the bond is.
On the other hand. In an ionic compound the number of cations and anions are equal. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated.
Fluorine is a non-metal so a bond between two will be covalent bond instead of an Ionic. Magnesium and Lithium are both metals so a bond will not be ionic. Ionic compounds consist of cations and anions whose total charges cancel each other out.
CaF2 is an ionic compound because in the CaF2 molecule calcium acts as a cation by donating its 2 extra electrons from the valence shell and Fluoride acts as an anion by. Compounds can be classified as ionic or covalent. If the electronegativity difference is less than 200 the bond is ionic.
22 ionic or covalent calculator. Bonds formed between a metal cation and a non-metal anion due to the electrostatic force that binds ions of opposite charge. CaF2 is an ionic compound because in the CaF2 molecule calcium acts as a cation by donating its 2 extra electrons from the valenc Sabtu 22.
If the electrons are shared equally between the atoms then its a non-polar covalent bond. To determine whether a bond is ionic or covalent you can calculate the electronegativity difference between 2 atoms.

Introduction To Chemistry General Organic And Biological Pdf Chemical Bond Ionic Bonding

0471770264 Pdf Acid Properties Of Water

Chapter 15 16 Bonding Ppt Video Online Download
Analytical Chem Istry Depauw University
What Is The Bond Order Of N2 Quora

Mercurous Ion Hg2 2 Pubchem

Pdf Abnormal Development Of Cone Cells In Transgenic Mice Ablated Of Rod Photoreceptor Cells Jiro Usukura Academia Edu

Monomeric And Trimeric Thorium Chlorides Isolated From Acidic Aqueous Solution Inorganic Chemistry

Browse Questions For Chemistry 101

Pdf Atkins Ravinesh Singh Academia Edu

Pdf Analytical Chemistry Laura G Anzaldo Academia Edu
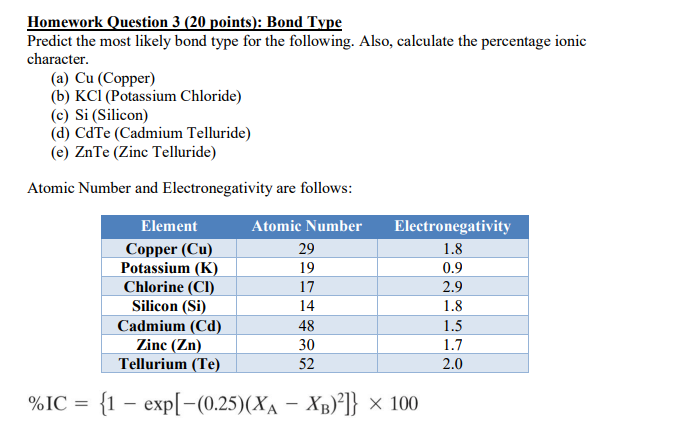
Solved Homework Question 3 20 Points Bond Type Predict Chegg Com

Lewis Structure Calculator Lewis Structure Generator
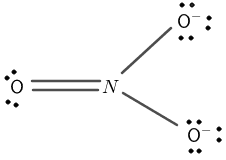
Lewis Structure Calculator Online Solver With Free Steps

Catalog 2010 2011 By Ben Bull Issuu

Physical Chemistry For The Life Sciences 2e Pdf Molecular Orbital Entropy

Key Strategy For The Rational Incorporation Of Long Lived Nir Emissive Cr Iii Chromophores Into Polymetallic Architectures Inorganic Chemistry